Immuno-oncology based research attempt to enable patients’ immune system against cancer cells. With the development, several immune checkpoint are found involving in the mechanism of immune evasion. The corresponding inhibitors, such as PD-L1 and CTLA-4 are applied to improve the prognosis of patients. To better evaluate the effects of these checkpoint inhibitors, tumour mutation burden (TMB) is involve in the treatment to evaluate the metric of non-silent somatic mutations per megabase of coding DNA.
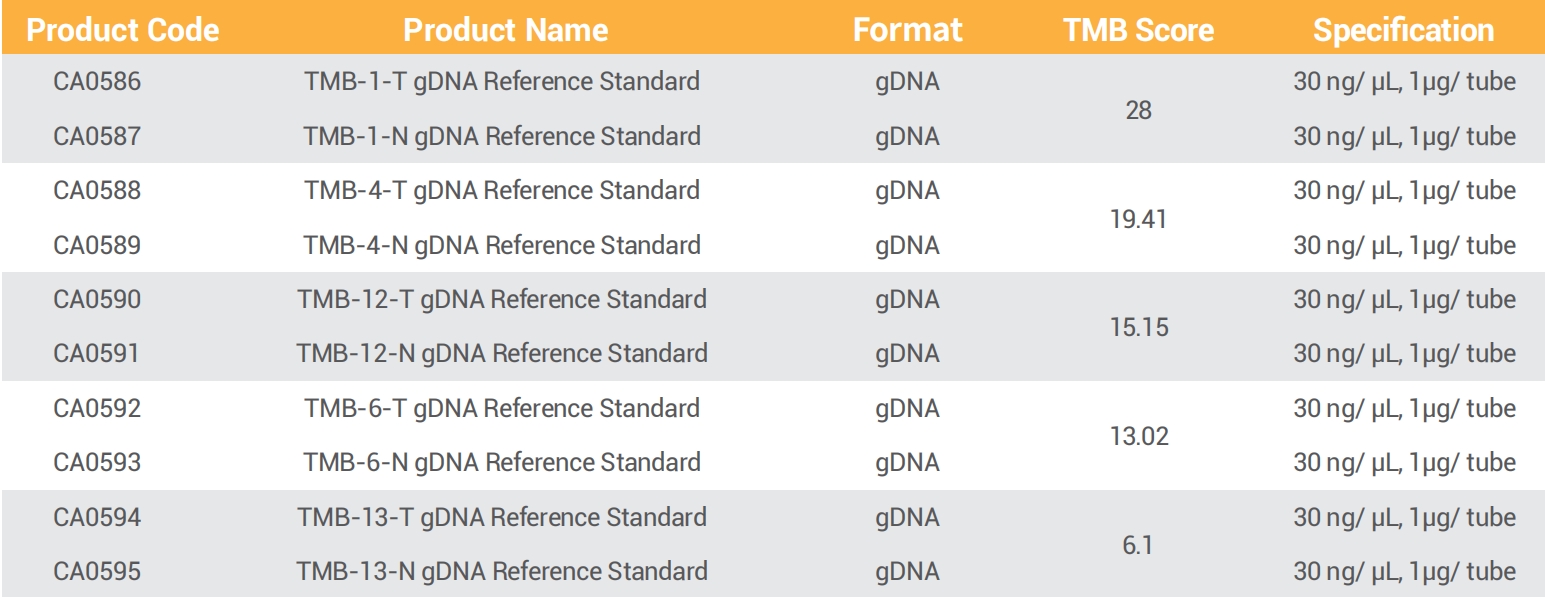
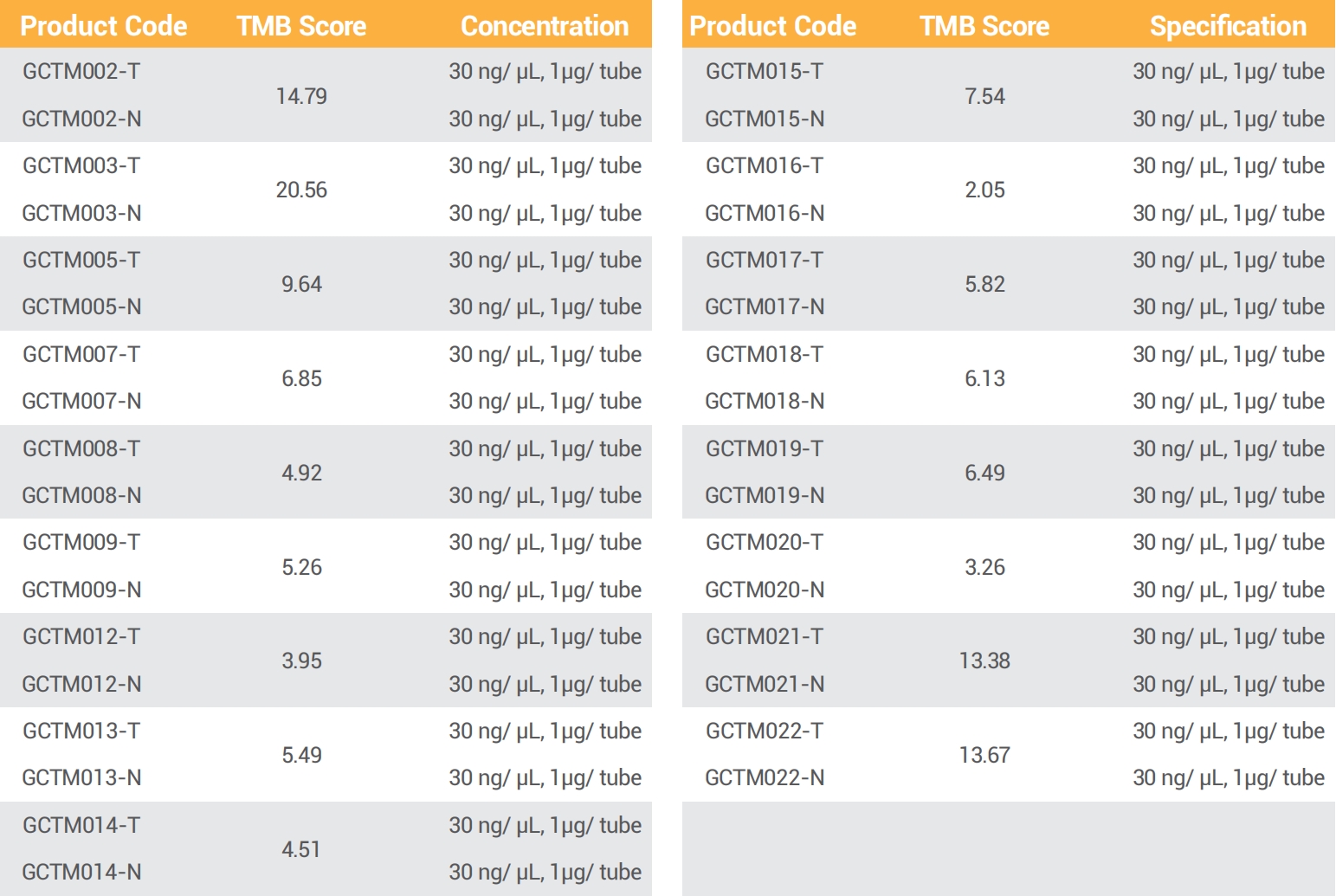
GeneWell developed tumour normal matched, genetically identified TMB reference standards, covering a variety of TMB scores. These reference standards are derived from human cell lines to mimic clinical samples and available in various format to act as a whole process quality control material.
● Tumor-normal matched reference standards
● Genomic DNA, ctDNA and FFPE formats are available
● ddPCR verified allelic frequency to determine the limit of detection
● Compatible for multiple CDx development application
● 22 pairs of TMB reference standards
● TMB scores range from 2 to 28
● Provide whole exome sequencing WES data of each batch, TMB score and bioinformatics pipeline
● Mimic clinical samples with desired tumour fraction by mixing purified tumour and normal DNA