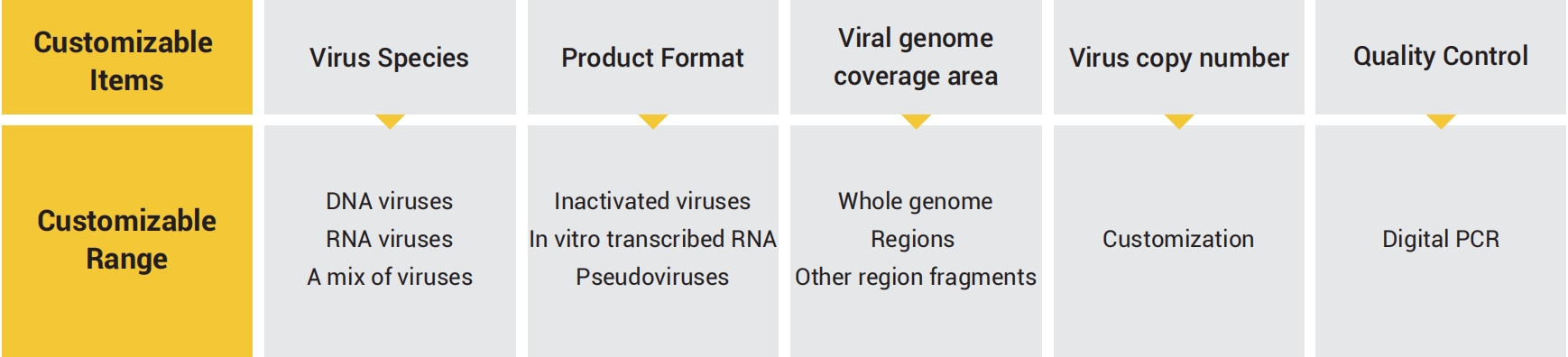
GeneWell has developed DNA and RNA virus quality controls according to the demand of consistency and accuracy of viral nucleic acid tests. Our quality controls can be customized in various formats, specifications and types of virus, specifically for your testing.
● Various formats
Inactivated virus: closest to clinical sampels, applicable for the quality controls of whole process of viral nucleic acid detection (including viral cleavage and nucleic acid extraction)
Pseudovirus: quality control for extraction step, plasmid DNA residue rate stable controlled at lower than 0.1% In vitro transcribed RNA: Accurate quality controls for quantitative and qualitative experimental processes, more stable for transportation and preservation
● Accurate quantification
Virus copy number quantification was performed using droplet digital PCR
● Flexible targets
Genome-wide or specific targets can be customized
● Applicable platform
PCR, NGS, POCT and other nucleic acid detection platform
● Registration certificate
Positive quality control products and enterprise reference products
● Routine quality control
Evaluate the stability and reliability of the test process
● Kits and platforms
Parallel comparison of test kit and external quality assessment
Click the product name to detail page.
| No. | Product Name | Product Code | Specification |
|---|---|---|---|
| 1 | Inactivated virus solutions | IB-GW-IAF101~IB-GW-IAF142 | 100 μL/ tube |