Non-invasive prenatal testing (NIPT) analyses fetal DNA present in maternal blood to screen for genetic disorders. With the development of Next Generation Sequencing (NGS) platforms, the screening targets has expended form common fetal aneuploidies to autosomal and sex chromosome aneuploidies, as well as some microdeletions, large copy number variants, and monogenic disorders.
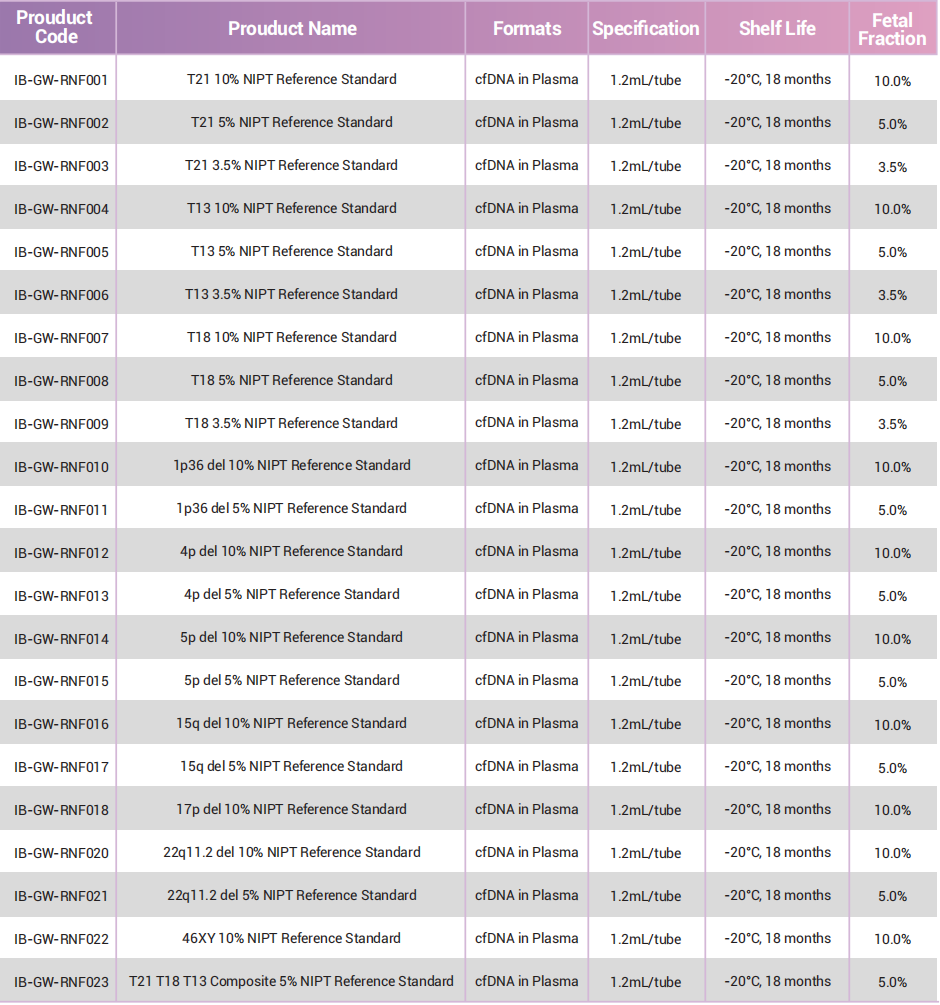
GeneWell NIPT Reference Standards contain fragmented human spiked into a commutable matrix (simulated human plasma). These reference standards are formulated to mimic patient samples and serve as excellent quality controls by acting as performance indicators for the entire NIPT diagnostic workflow including sample extraction, library preparation and sequencing. This product is designed to be compatible with multiple platforms, including shotgun massively parallel sequencing, targeted massively parallel sequencing and single nucleotide polymorphism methods.
● Entire workflow quality control
Reference standards are formulated in human plasma matrix which mimicing patient samples and monitoring the entire workflow from extraction.
● Compatible with multiple NIPT assay platforms
Matched or simulated maternal-fetal reference standards suit a broad range of NIPT assays such as massively parallel sequencing and single nucleotide polymorphism methods.
● Comprehensive coverage of variants for workflow performance evaluation
The Reference panel covers common chromosomal abnormalities as well as pathogenic copy number variations ranging from 2Mb to 30 Mb.
● Flexible portfolio of fetal fraction levels
Fetal DNA ratio can be customised to match your assay and to determine the limit of detection.