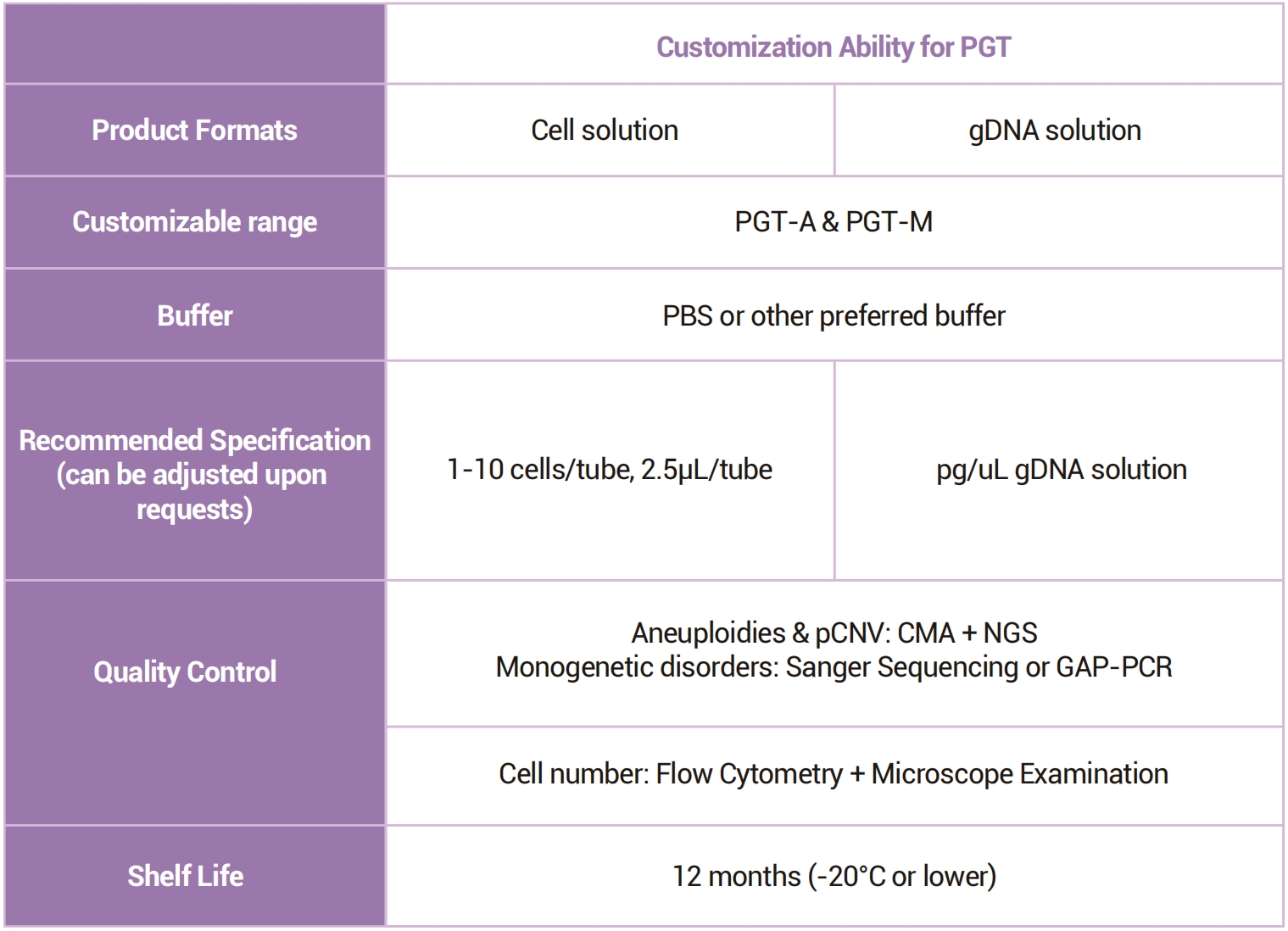
Preimplantation genetic testing (PGT) is an early form of prenatal genetic diagnosis where abnormal embryos are identified, thereby allowing transfer of genetically normal embryos. Combination with the resource of NIPT and inherited diseases cell lines, GeneWell offers a unique portfolio of patient sample liked PGT-A and PGT-M reference standards in cell solution format, at 1 to 10 cell per tube. They mostly mimic the status of clinical samples in division period of zygote, which are ideal for the whole process quality control and performance validation of PGT testing in aneuploidy and monogenic disorders. Additionally, these PGT reference standards can also be customised in various format and concentration to further convenience the usage.
● Comprehensive Coverage
PGT-A can be customised from common Trisomy 13/18/21 to rare pCNV variants. PGT-M includes a wide range of inherited genetic diseases.
● Consistency and Accuracy
Offering chromosomal microarray analysis results of cell lines.
● Wide Compatibility
Compatible with both NGS and array-based PGT assays.
● Mimic clinical samples
Low concentrations of gDNA or small amounts of cells in buffers mimic clinical samples in IVT, ideally to be used as external quality controls for the whole testing process.
● Highly customizable
Besides aneuploidies and monogenic disorder samples, multiplex reference standards and simulated variant fractions can also be customised upon request.
More samples are avaliable for PGT customization, please contact us for any enquires!